Popular Products
30ml Bacteriostatic Water for Injection (each)
- $16.51
$0.00- $16.51
- Unit price
- / per
- $16.51
$0.00- $16.51
- Unit price
- / per
30ml Bacteriostatic Water for Injection (3 Pack)
- $47.53
$50.85- $47.53
- Unit price
- / per
- $47.53
$50.85- $47.53
- Unit price
- / per
30ml Bacteriostatic Water for Injection (5 Pack)
- $72.55
$84.75- $72.55
- Unit price
- / per
- $72.55
$84.75- $72.55
- Unit price
- / per
Sterile Water for Injection, USP 10 mL (1 bottle)
- $8.73
- $8.73
- Unit price
- / per
- $8.73
- $8.73
- Unit price
- / per
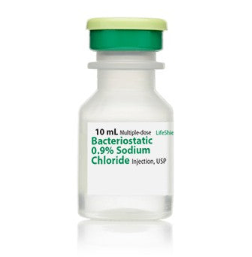





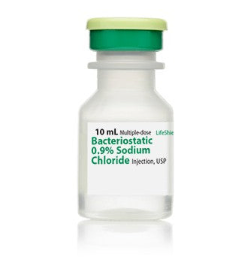
⏳Bacteriostatic 0.9% Sodium Chloride Injection, USP (10 mL bottle)
- $6.84
$10.84- $6.84
- Unit price
- / per
Free Shipping
Free standard shipping on orders over $199
SHIPPING NOTIFICATION
Holiday Shipping Schedule — 2025
Peak-season volumes may delay regular ground shipments. Time-definite services and pickup dates are below.
Service Alerts
- Ground Advantage is currently 3–5 business days.
- Delivery dates shown are ESTIMATED, not guaranteed.
- For guaranteed delivery, select UPS Next Day Air® or USPS Priority Express.
- Saturday delivery available in certain locations only.
Note: USPS Ground Advantage can take 7–9 business days to some ZIP codes—choose expedited shipping if you’re on a deadline.
Thanksgiving
Note: Due to “peak season”, regular ground shipping may experience delays.
- Wednesday, Nov 26 — All UPS Next Day Air® and USPS Priority Express Mail picked up Nov 26 are scheduled for delivery on Friday, Nov 28.
- Wednesday, Nov 26 — UPS 2nd Day Air® picked up Nov 26 is scheduled for delivery on Monday, Dec 1 (except those processed/labeled for Saturday, Nov 29 — additional fee required).
- Thursday, Nov 27 — CLOSED
- Friday, Nov 28 — CLOSED
Christmas
Note: Due to “peak season”, regular ground shipping may experience delays.
- Monday, Dec 22 — Last day to ship UPS 2nd Day Air® for delivery on Wednesday, Dec 24. UPS 3 Day Select® picked up Dec 22 is scheduled for delivery on Friday, Dec 26.
- Tuesday, Dec 23 — Last day to ship UPS Next Day Air® for delivery on Wednesday, Dec 24.
- Wednesday, Dec 24 (Christmas Eve) — CLOSED
- Thursday, Dec 25 — CLOSED
Description
xEXPIRATION DATE: 31 OCT 2026
OUR loss is YOUR gain!
We received a limited amount of bacteriostatic sodium chloride with a short shelf life from our distributor, so we're offering these bottles at a reduced rate. Save $4.00 and use before expiring.
This preparation is designed for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection.
Bacteriostatic 0.9% Sodium Chloride Injection, USP is a sterile, non-pyrogenic, isotonic solution of sodium chloride in water for injection. Each milliliter (mL) contains sodium chloride 9 mg and 0.9% (9 mg/mL) benzyl alcohol added as a bacteriostatic preservative. May contain hydrochloric acid for pH adjustment.
It is supplied in a multiple-dose container from which repeated withdrawals may be made to dilute or dissolve drugs for medication. The pH is 5.0 (4.5 to 7.0).
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
Related Products
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- $6.84
$10.84- $6.84
- Unit price
- / per
- Choosing a selection results in a full page refresh.