Free Shipping over $199 -
Use new customer discount code NEWCUST at checkout for 10% off your entire order!
Popular Products
30ml Bacteriostatic Water for Injection (each)
- $16.95
$0.00- $16.95
- Unit price
- / per
* Limited Inventory as of December 2025 Expiration Date: 28 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must...
- $16.95
$0.00- $16.95
- Unit price
- / per
30ml Bacteriostatic Water for Injection (3 pack)
- $48.15
$50.85- $48.15
- Unit price
- / per
Expiration Date: 28 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior...
- $48.15
$50.85- $48.15
- Unit price
- / per
30ml Bacteriostatic Water for Injection (5 Pack)
- $79.05
$84.75- $79.05
- Unit price
- / per
Expiration Date: 28 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior...
- $79.05
$84.75- $79.05
- Unit price
- / per
Sterile Water for Injection, USP 10 mL (1 bottle)
- $8.73
- $8.73
- Unit price
- / per
EXPIRATION DATE: On Manufacturer Backorder Brand: Hospira Dispensed in a 10mL, plastic, single-dose vial. This preparation is designed solely for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection. Sterile Water for Injection,...
- $8.73
- $8.73
- Unit price
- / per
SKU:
SV5 | 6332300105
Availability:
In Stock
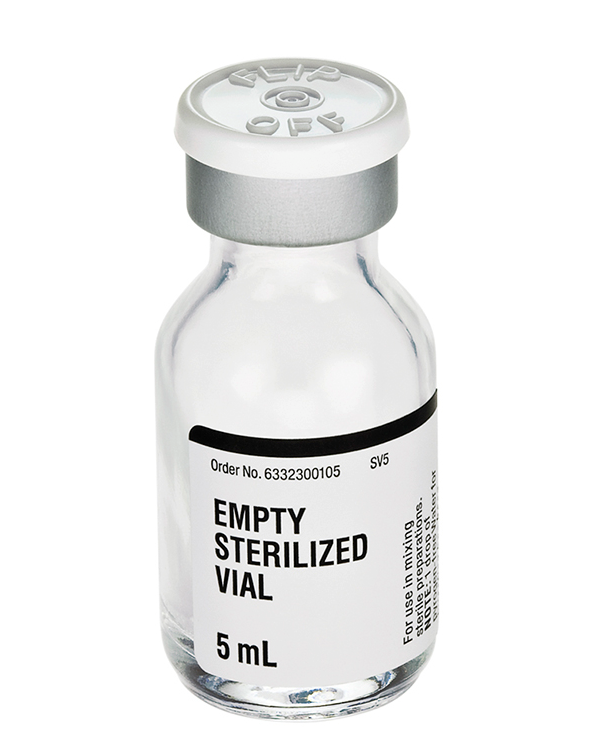
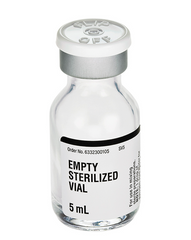
Empty Sterilized Vial - Glass (Fresenius Kabi) 5mL
- $5.08
$0.00- $5.08
- Unit price
- / per
Manufactured by Fresenius Kabi Labs EXP Date: 1 OCT 2027 For use in mixing sterile preparations. Empty...
Subtotal:
$5.08
10 customers are viewing this product
Free Shipping
Free standard shipping on orders over $199
SHIPPING NOTIFICATION
Holiday Shipping Schedule — 2025
Peak-season volumes may delay regular ground shipments. Time-definite services and pickup dates are below.
Service Alerts
- Ground Advantage is currently 3–5 business days.
- Delivery dates shown are ESTIMATED, not guaranteed.
- For guaranteed delivery, select UPS Next Day Air® or USPS Priority Express.
- Saturday delivery available in certain locations only.
Note: USPS Ground Advantage can take 7–9 business days to some ZIP codes—choose expedited shipping if you’re on a deadline.
Thanksgiving
Note: Due to “peak season”, regular ground shipping may experience delays.
- Wednesday, Nov 26 — All UPS Next Day Air® and USPS Priority Express Mail picked up Nov 26 are scheduled for delivery on Friday, Nov 28.
- Wednesday, Nov 26 — UPS 2nd Day Air® picked up Nov 26 is scheduled for delivery on Monday, Dec 1 (except those processed/labeled for Saturday, Nov 29 — additional fee required).
- Thursday, Nov 27 — CLOSED
- Friday, Nov 28 — CLOSED
Christmas
Note: Due to “peak season”, regular ground shipping may experience delays.
- Monday, Dec 22 — Last day to ship UPS 2nd Day Air® for delivery on Wednesday, Dec 24. UPS 3 Day Select® picked up Dec 22 is scheduled for delivery on Friday, Dec 26.
- Tuesday, Dec 23 — Last day to ship UPS Next Day Air® for delivery on Wednesday, Dec 24.
- Wednesday, Dec 24 (Christmas Eve) — CLOSED
- Thursday, Dec 25 — CLOSED
Description
xManufactured by Fresenius Kabi Labs
EXP Date: 1 OCT 2027
For use in mixing sterile preparations.
Empty Glass Vials Features:
- 5 mL, 16.5 mm closure
- The container closure is not made with natural rubber latex.
- One drop of pyrogen-free water has been added to the vial for sterilization during autoclaving.
- Protective flip top lid to be removed prior to use
Related Products
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
Westend Medical
Example product title
- $5.08
$0.00- $5.08
- Unit price
- / per
- Choosing a selection results in a full page refresh.