Popular Products
30ml Bacteriostatic Water for Injection (each)
- $16.51
$0.00- $16.51
- Unit price
- / per
- $16.51
$0.00- $16.51
- Unit price
- / per
30ml Bacteriostatic Water for Injection (3 Pack)
- $47.53
$50.85- $47.53
- Unit price
- / per
- $47.53
$50.85- $47.53
- Unit price
- / per
30ml Bacteriostatic Water for Injection (5 Pack)
- $72.55
$84.75- $72.55
- Unit price
- / per
- $72.55
$84.75- $72.55
- Unit price
- / per
Sterile Water for Injection, USP 10 mL (1 bottle)
- $8.73
- $8.73
- Unit price
- / per
- $8.73
- $8.73
- Unit price
- / per
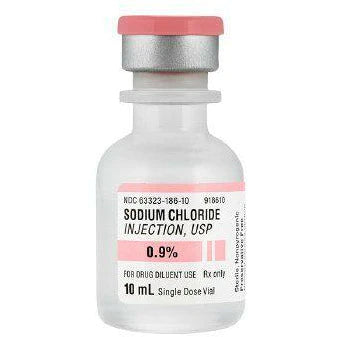


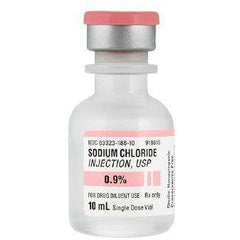

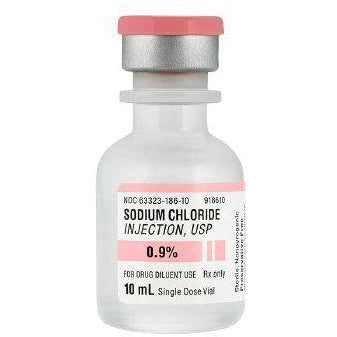
Sodium Chloride Injection, USP 0.9% Fresenius (10 mL bottle)
- $5.83
$14.95- $5.83
- Unit price
- / per
Free Shipping
Free standard shipping on orders over $199
SHIPPING NOTIFICATION
Holiday Shipping Schedule — 2025
Peak-season volumes may delay regular ground shipments. Time-definite services and pickup dates are below.
Service Alerts
- Ground Advantage is currently 3–5 business days.
- Delivery dates shown are ESTIMATED, not guaranteed.
- For guaranteed delivery, select UPS Next Day Air® or USPS Priority Express.
- Saturday delivery available in certain locations only.
Note: USPS Ground Advantage can take 7–9 business days to some ZIP codes—choose expedited shipping if you’re on a deadline.
Thanksgiving
Note: Due to “peak season”, regular ground shipping may experience delays.
- Wednesday, Nov 26 — All UPS Next Day Air® and USPS Priority Express Mail picked up Nov 26 are scheduled for delivery on Friday, Nov 28.
- Wednesday, Nov 26 — UPS 2nd Day Air® picked up Nov 26 is scheduled for delivery on Monday, Dec 1 (except those processed/labeled for Saturday, Nov 29 — additional fee required).
- Thursday, Nov 27 — CLOSED
- Friday, Nov 28 — CLOSED
Christmas
Note: Due to “peak season”, regular ground shipping may experience delays.
- Monday, Dec 22 — Last day to ship UPS 2nd Day Air® for delivery on Wednesday, Dec 24. UPS 3 Day Select® picked up Dec 22 is scheduled for delivery on Friday, Dec 26.
- Tuesday, Dec 23 — Last day to ship UPS Next Day Air® for delivery on Wednesday, Dec 24.
- Wednesday, Dec 24 (Christmas Eve) — CLOSED
- Thursday, Dec 25 — CLOSED
Description
xEXPIRATION DATE: 01 FEB 2027
Manufacturer: Fresenius Kabi USA
This parenteral preparation is indicated only for diluting or dissolving drugs for intravenous, intramuscular, or subcutaneous injection.
Sodium Chloride injection, USP, 0.9% is a sterile, non-pyrogenic solution. Each mL contains: Sodium Chloride 9mg; Water for injection. It contains no bacteriostat, anti-microbial agent, or added buffer and is supplied only in single dose containers. Discard any unused portion.
Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment (pH 4.5-7.0).
Storage: Store at controlled room temperature 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature]. Do not freeze.
-----------------
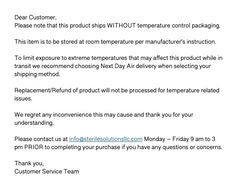
Please note that this product ships WITHOUT temperature control packaging.
This item is to be stored at room temperature per manufacturer’s instruction.
To limit exposure to extreme temperatures that may affect this product while in transit we recommend choosing Next Day Air delivery when selecting your shipping method.
- Replacement/Refund of product will not be processed for temperature related issues.
- We regret any inconvenience and thank you for your understanding.
- Please contact us at info@sterilesolutionsllc.com prior to completing your purchase if you have any questions or concerns.
Thank You
Customer Service Hours
Monday – Friday
9 am to 3 pm CST
Related Products
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- $5.83
$14.95- $5.83
- Unit price
- / per
- Choosing a selection results in a full page refresh.